Explain why caprylic acid, C  (C
(C 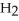
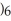 COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C
COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C 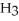 (C
(C 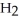
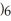 CHO, does not.
CHO, does not.
Definitions:
Civilize
To bring a society to a stage of social, cultural, and moral development considered to be more advanced, often used historically in a context of colonialism and imperialism.
Equal Rights
The principle or aim of ensuring all individuals are granted the same freedoms and legal protections, regardless of gender, race, or other characteristics.
Unsuccessful Effort
An attempt or endeavor that fails to achieve its intended outcome or goal.
Audience
A group of listeners or viewers who participate in experiencing or attending an event, such as a performance, lecture, or broadcast.
Q1: Carbon dioxide reacts with water to form
Q14: In your travels through space, if you
Q18: The general outcome of anabolism is _.<br>A)
Q35: What is the percent volume of water
Q48: Which of the following items would you
Q65: Which of the following is an organic
Q83: Which is better for you: a drug
Q106: What is the purpose of an enzyme?<br>A)
Q130: In a still room, cigar smoke sometimes
Q147: Which of the following two stick structures