Explain why caprylic acid, C  (C
(C 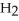
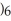 COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C
COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C 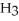 (C
(C 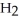
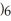 CHO, does not.
CHO, does not.
Definitions:
Syndesmoses
A type of fibrous joint in which bones are joined together by a ligament or a fibrous tissue, allowing for minimal movement.
Gomphoses
A type of fibrous joint that is a peg-in-socket, such as the joint between a tooth and its socket in the jaw.
Hip And Shoulder
Joints that connect the limbs to the torso; the hip being the articulation between the pelvis and the femur, and the shoulder connecting the upper arm to the torso.
Gliding Motion
A smooth movement where surfaces slide past one another, typical in some types of joints like the carpals of the wrist.
Q4: What polypeptide is coded for by the
Q30: Which of the following pathways of the
Q41: Most fresh water lakes _.<br>A) contain no
Q54: What would the concentration of OH<sup>-</sup> be
Q83: Which element is closer to the upper
Q83: What do all fats have in common?<br>A)
Q124: The fungus causing Dutch Elm disease is
Q143: How close are you to a CFC
Q143: Which natural law is illustrated by the
Q156: If an alcoholic beverage has a proof