An ideal gas undergoes the process a→b→c→a shown in the pV diagram. The heat gained by the gas in process a→b is 546 J, while in process the gas loses 62.0 J of heat. In process a→b the gas performs of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a? 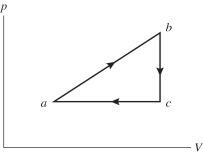
Definitions:
Part Name
The designated name given to a specific component within an assembly or system.
Date Of Drawing
The specific date when a drawing or blueprint was created or last revised.
Metric Drawing
A type of technical drawing in which dimensions and measurements are expressed in metric units, such as millimeters and meters.
Lettering
The act or process of marking or inscribing with letters, especially in a specific style or font in technical documentation.
Q12: A polar bear of mass 200 kg
Q31: An object undergoing simple harmonic motion
Q31: A 15-kg child is sitting on a
Q51: A block of mass m =
Q85: A golf ball of mass 0.050
Q143: A solid uniform ball of mass 1.0
Q149: It requires 0.30 kJ of work
Q159: A container of 114.0 g of
Q166: A typical incandescent light bulb consumes 75
Q173: The figure shows a pV diagram for