A substance has a melting point of 20°C and a heat of fusion of 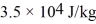 .The boiling point is 150°C and the heat of vaporization is
.The boiling point is 150°C and the heat of vaporization is 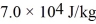 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 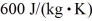 ,
, 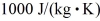 ,and
,and 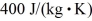 ,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
Definitions:
Process Time
The time required to complete a particular process, often including the steps of production or task execution.
Bottleneck Management
The process of identifying, managing, and mitigating bottlenecks or points of congestion that slow down production or workflow in an operation.
Work Orders
A formal document that outlines the details for completing a specific task or job, typically including what needs to be done, by whom, and by when.
System Capacity
The maximum output or activity level that a system can achieve under normal or specified conditions.
Q14: If the electric potential in a region
Q27: Three capacitors,of capacitance 5.00 μF,10.0 μF,and 50.0
Q32: An adiabatic compression is performed on an
Q36: A 72.0-kg person pushes on a small
Q41: A container is filled with a mixture
Q46: The voltage and power ratings of a
Q47: A mole of oxygen (O<sub>2</sub>)molecules and a
Q48: A uniform solid sphere is rolling without
Q49: A nuclear fission power plant has an
Q53: A 1.86-kg block is held in place