How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 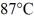 to produce water at a final temperature of
to produce water at a final temperature of 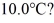 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 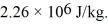
Definitions:
Poisoning
A condition or physical state caused by the ingestion, inhalation, injection, or skin absorption of toxic substances.
Suicide-Focused Forums
Online platforms or communities where individuals discuss topics related to suicide, which can range from seeking support to sharing suicidal ideations.
Suicide Prevention Centers
Organizations dedicated to providing support and resources to prevent suicide and support those affected by suicidal thoughts.
Hotlines
Dedicated telephone or online services that provide immediate support and resources for people in distress.
Q7: For the apparatus shown in the figure,there
Q28: A mass M is attached to an
Q28: A transverse wave is traveling on a
Q33: A long,thin rod parallel to the y-axis
Q34: An ideal gas is compressed in a
Q37: The cube of insulating material shown in
Q38: Three solid,uniform,cylindrical flywheels,each of mass 65.0 kg
Q38: Suppose you have two negative point charges.As
Q40: Water flows through a pipe having a
Q50: A cable is 100 m long,has a