How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 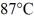 to produce water at a final temperature of
to produce water at a final temperature of 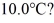 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg • C° and of ice is 2050 J/kg • C°.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 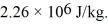
Definitions:
Social-psychological Theory
A framework that explores how individual behavior is influenced by social interaction and the presence of other people.
Prohibition
The legal act of forbidding something, especially the manufacture, transportation, and sale of alcoholic beverages in historical contexts.
Constitutional Amendment
A formal change or addition proposed or made to a constitution.
Social Consequences
The effects of actions, behaviors, or policies on a society or community, including impacts on social structures and relationships.
Q10: Two compressible solids are formed into spheres
Q15: What is the radius of a sphere
Q18: A 30.0-kg child sits on one end
Q29: A 2.50-kg stone is dropped from rest
Q32: When two or more capacitors are connected
Q34: A 12-L volume of oil is subjected
Q40: There must be equal amounts of mass
Q41: Shock waves occur when<br>A) the frequency of
Q44: An engine manufacturer makes the claim that
Q52: In the figure,all the charges are point