A 3.2-L volume of neon gas (Ne) is at a pressure of 3.3 atm and a temperature of 330 K.The atomic mass of neon is 20.2 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol,and the ideal gas constant is 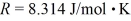 =
= 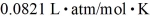 .The mass of the neon gas is closest to
.The mass of the neon gas is closest to
Definitions:
Frictional Unemployment
Short-term unemployment that arises from the process of matching workers with jobs.
Workers Equally Well Suited
A situation in which workers possess the same skills and abilities, making them interchangeable in their roles without affecting productivity.
Structural Unemployment
A form of unemployment caused by a mismatch between the skills that workers in the economy can offer and the skills demanded by employers.
Supply And Demand Equilibrium
The point at which the quantity of a product demanded by consumers equals the quantity supplied by producers.
Q7: Electric charge is uniformly distributed inside a
Q11: A 8.0-m long wire with a mass
Q14: Two automobiles traveling at right angles to
Q15: The graph in the figure shows the
Q22: The moons of Mars,Phobos (Fear)and Deimos (Terror),are
Q30: An object is undergoing simple harmonic motion
Q32: An adiabatic compression is performed on an
Q39: A 6.0-m wire with a mass of
Q43: The amplitude of a lightly damped harmonic
Q61: A certain source of sound waves radiates