An ideal gas is at a pressure 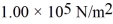 and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
Definitions:
Fixed Costs
Fixed costs are business expenses that remain constant regardless of the level of production or sales activities, such as rent, salaries, and insurance.
Contribution Margin
The sales price minus the variable costs of a product, indicating how much selling one more unit adds to profit.
Variable Costs
Costs that vary directly with the level of production or volume of output, such as raw materials and direct labor.
Fixed Costs
Costs that remain constant regardless of the volume of goods or services produced by a business.
Q8: In the figure,when the terminal voltage V<sub>ab</sub>
Q9: An L-shaped metal machine part is made
Q17: Each plate of an air-filled parallel-plate air
Q19: What is the maximum theoretical efficiency possible
Q24: A gold wire that is 1.8 mm
Q28: A 2.0 mm diameter wire of length
Q33: The density of aluminum is 2700 kg/m<sup>3</sup>.If
Q35: A 310-g air track cart is traveling
Q39: Consider a spherical Gaussian surface of radius
Q54: The figure shows a 2.0-cm diameter roller