Multiple Choice
A sealed container holds 0.020 moles of nitrogen (N2) gas at a pressure of 1.5 atmospheres and a temperature of 290 K.The atomic mass of nitrogen is 14 g/mol.The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is 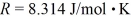 =
= 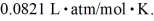 The average translational kinetic energy of a nitrogen molecule is closest to
The average translational kinetic energy of a nitrogen molecule is closest to
Definitions:
Related Questions
Q7: A straight 15.0-g wire that is 2.00
Q9: A certain planet has an escape speed
Q14: An ideal massless spring with a spring
Q17: Charge is distributed uniformly throughout a large
Q19: For the circuit shown in the figure,what
Q26: A piece of thin uniform wire of
Q32: A charge Q = -820 nC is
Q32: When two or more capacitors are connected
Q43: During an adiabatic process,an ideal gas does
Q44: The position x of an object varies