What is the mean free path for the molecules in an ideal gas when the pressure is 100 kPa and the temperature is 300 K given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? The Boltzmann constant is 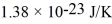 ,Avogadro's number is
,Avogadro's number is 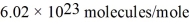 ,and the ideal gas constant is
,and the ideal gas constant is 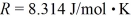 =
= 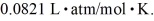
Definitions:
Uncertainty Reduction
The process of lessening ambiguity in communicating, often by gathering more information about others.
Social Support
The assistance and protection provided by others, especially through social relationships and networks.
Cultivate Attraction
Deliberately developing or increasing feelings of interest or appeal towards someone or something.
Socially Decenter
The ability to step outside one's own perspective to understand the thoughts and feelings of others.
Q4: A sound source emits 20.0 W of
Q8: Consider the circuit shown in the figure.Note
Q15: A solid steel sphere with a radius
Q22: An organ pipe open at both ends
Q24: What is the net power that a
Q32: An object is executing simple harmonic motion.What
Q39: In a certain region,the electric potential due
Q43: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="A sealed
Q45: A perfect Carnot engine operates between the
Q48: A steel guitar string with a diameter