On a hot summer day,the temperature is 40.0°C and the pressure is 1.01 × 105 Pa.Let us model the air as all nitrogen of molecular mass 28.0 g/mol having molecules of diameter 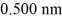 that are moving at their root-mean-square speed.Avogadro's number is
that are moving at their root-mean-square speed.Avogadro's number is 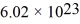 molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is
molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is 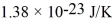 .Calculate reasonable estimates for
.Calculate reasonable estimates for
(a)the root-mean-square speed of the nitrogen molecules.
(b)the average distance a typical molecule travels between collisions.
(c)the average time a molecule travels between collisions,assuming that the molecules are moving at their root-mean-square speeds.
(d)the number of collisions an average molecule undergoes per second.
Definitions:
Incremental Overhead
Additional overhead costs that are incurred when an organization increases its production volume or undertakes new activities.
Financing Costs
Expenses incurred by a company in the process of raising capital through debts and/or equity, including interest payments and commissions.
Basic Overheads
The regular and necessary costs, such as rent and utilities, that are involved in operating a business.
Interest Charges
This is the cost incurred for borrowing money, typically expressed as a percentage of the principal loan amount.
Q3: The figure shows the displacement y of
Q10: As the speed of a moving fluid
Q18: A parallel-plate capacitor has plates of area
Q20: A cubic box with sides of 20.0
Q22: A 7.8-kg solid sphere,made of metal whose
Q28: A tire is rolling along a road,without
Q37: Two capacitors,C<sub>1</sub> and C<sub>2</sub>,are connected in series
Q39: The figure shows three long,parallel,current-carrying wires.The current
Q40: A conducting sphere contains positive charge distributed
Q46: The voltage and power ratings of a