What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is 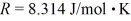 =
= 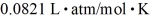 .
.
Definitions:
Virtual Destruction
The act of causing significant damage or annihilation in a digital or simulated environment.
Western Conceptions
Ideas, beliefs, or values that originated or are predominantly held in Western societies.
Reasoning
The process of thinking about something in a logical, rational way to form a conclusion or judgment.
Sets Of Premises
Groups of statements or propositions that provide the basis for a conclusion in an argument.
Q3: A proton is placed in an electric
Q13: A charged particle of mass 0.0020 kg
Q15: When a fixed amount of ideal gas
Q20: For the circuit shown in the figure,determine
Q29: The point charge at the bottom of
Q30: A 3.0-μC positive point charge is located
Q32: At a certain depth in the ocean,the
Q34: The diameter of a 12-gauge copper wire
Q39: A string is wrapped around a pulley
Q63: The walls of an ice chest are