During an adiabatic process,20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa.The ideal gas constant is 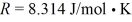 .
.
(a)What is the final volume of the gas?
(b)How much heat does the gas exchange during this process?
(c)What is the change in the internal (thermal)energy of the gas during this process?
Definitions:
TRIN Statistic
A technical analysis indicator known as the TRading INdex (TRIN), which compares the number of advancing and declining stocks to their respective volume, aiming to gauge overall market sentiment.
Bearish Signal
An indication in technical analysis or market behavior suggesting that the price of an asset is likely to decline.
Bullish Signal
An indicator suggesting that the price of an asset is likely to increase, often leading investors to buy or hold positions.
Relative Strength
A momentum investing technique comparing the performance of a stock or asset to that of a broader market index to gauge its strength.
Q3: Three very long,straight,parallel wires each carry currents
Q8: A charged capacitor stores energy U.Without connecting
Q9: A 0.025-kg block on a horizontal frictionless
Q19: A steel lift column in a service
Q21: An air-filled capacitor is formed from two
Q22: A Carnot refrigerator takes heat from water
Q22: An organ pipe open at both ends
Q32: As shown in the figure,a wire and
Q45: A child is trying to stack two
Q46: A metal cylinder of radius 2.0 mm