An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings.How much work is done by the gas if the outside pressure is slowly reduced,allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 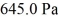 and a volume of
and a volume of 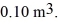 The ideal gas constant is R = 8.314 J/mol • K.
The ideal gas constant is R = 8.314 J/mol • K.
Definitions:
Coenzyme
An organic cofactor.
Flavine Adenine Dinucleotide
A cofactor involved in various metabolic processes, essential in energy production from nutrients.
Nicotine Adenine Dinucleotide Phosphate
A form of NADP, a coenzyme involved in redox reactions, playing crucial roles in anabolic processes and the oxidative phase of photosynthesis.
Cofactors
Chemical compounds or metallic ions not made of protein, essential for the catalytic activity of enzymes.
Q5: Find the speed of an ocean wave
Q6: A wheel has a radius of 0.40
Q8: A monatomic ideal gas undergoes an isothermal
Q18: A parallel-plate capacitor has plates of area
Q30: Two identical loudspeakers that are 5.00 m
Q37: When a potential difference of 10 V
Q40: A heat engine with an efficiency of
Q46: A 50-cm<sup>3</sup> block of wood is floating
Q47: In the circuit shown in the figure,all
Q61: Under steady state conditions,a piece of wood