During an adiabatic process,20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa.The ideal gas constant is 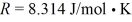 .
.
(a)What is the final volume of the gas?
(b)How much heat does the gas exchange during this process?
(c)What is the change in the internal (thermal)energy of the gas during this process?
Definitions:
Go Long
An investment strategy where an investor buys shares or securities with the expectation that their price will rise over time.
Sell Short
The sale of a security that the seller does not own at the time of the sale, typically with the intention of buying it back later at a lower price to make a profit.
Exchange Rates
The value of one currency for the purpose of conversion to another.
Consumer Price Index
A measure that examines the weighted average of prices of a basket of consumer goods and services, such as transportation, food, and medical care, commonly used as an indicator of inflation.
Q1: A +4.0 μC-point charge and a -4.0-μC
Q3: A conducting sphere is charged up such
Q6: In the figure,two solenoids are approaching each
Q11: Which contains more moles of material: 80
Q16: A jet aircraft,in level flight at constant
Q17: The intensity of sunlight falling on the
Q28: The charge on the square plates of
Q29: The speed of sound in the air
Q41: A quantity of ideal gas requires 800
Q48: An airplane flying faster than the speed