The figure shows the pV diagram for a certain thermodynamic process.In this process, 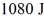 of heat flows into a system,and at the same time the system expands against a constant external pressure of
of heat flows into a system,and at the same time the system expands against a constant external pressure of 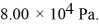 If the volume of the system increases from
If the volume of the system increases from 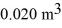 to
to 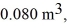 calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal)energy of the system.If the internal (thermal)energy change is nonzero,be sure to indicate whether this energy change is positive or negative. 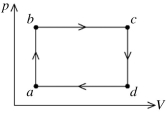
Definitions:
Directly Tipped Employee
An employee who receives tips directly from customers for services rendered, often subject to different tax reporting requirements.
Form 8027
A tax form used by employers who operate large food or beverage establishments to report tips received by employees.
FICA Tax
Federal Insurance Contributions Act tax, a payroll tax deducted from employees' wages for Social Security and Medicare benefits.
Employer
An individual or business entity that hires and pays wages or salaries to one or more employees in exchange for their work.
Q1: An ideal gas with γ = 1.30
Q4: An ideal gas is at a pressure
Q4: A Carnot engine operates between reservoirs at
Q13: If the current density in a wire
Q23: A man-made satellite of mass 6105 kg
Q34: A non-conducting sphere of radius R =
Q38: A Carnot cycle engine operates between a
Q39: An ideal parallel-plate capacitor consists of a
Q48: When a fixed amount of ideal gas
Q57: A hot air balloon has a volume