A cylinder contains 1.2 moles of ideal gas,initially at a temperature of 116°C.The cylinder is provided with a frictionless piston,which maintains a constant pressure of 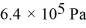 on the gas.The gas is cooled until its temperature has decreased to
on the gas.The gas is cooled until its temperature has decreased to 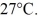 For the gas
For the gas 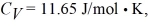 and the ideal gas constant
and the ideal gas constant 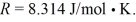 (a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(b)What is the change in the internal (thermal)energy of the gas during this process?
(c)How much heat is transferred to (or from)the gas during this process? Does this heat flow into or out of the gas?
Definitions:
Communication Strategy
A plan outlining how to effectively convey messages to targeted audiences through various channels to achieve specific objectives.
Office Systems
The combination of equipment, procedures, and technology used to facilitate work in an office environment.
Technical Support
A service provided to help users solve technical problems or issues related to products or services.
Inappropriate Tweet
A post on Twitter that is considered unsuitable or offensive, often leading to negative reactions.
Q10: An air-filled capacitor stores a potential energy
Q11: A uniform 300-kg beam,6.00 m long,is freely
Q13: If you double the pressure on the
Q20: Water flows in the horizontal pipe shown
Q25: A brass rod is 40.1 cm long
Q31: A sealed container holds 0.020 moles of
Q38: A rigid rectangular loop,which measures 0.30 m
Q41: A quantity of ideal gas requires 800
Q45: You are driving along a highway at
Q51: A weather balloon contains 12.0 m<sup>3</sup> of