An ideal Carnot engine operates between reservoirs having temperatures of 125°C and
-20°C.Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C.The heat of fusion of water is 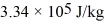 and the heat of vaporization of water is
and the heat of vaporization of water is 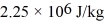 .
.
(a)How much work does this engine do each cycle?
(b)How much heat per cycle does this engine absorb at the hot reservoir?
Definitions:
Hazardous Occupations
Jobs or professions that inherently involve significant risks to the safety, health, or well-being of the workers.
Product Market
The marketplace where final goods or services are offered to consumers, businesses, and other entities.
Cost of Labor
The total amount spent by employers to compensate employees, including wages, benefits, and any other costs associated with employment.
Serve The Same Customers
The practice of targeting and catering to the same client base by different businesses or services.
Q2: The two water reservoirs shown in the
Q16: A dump truck has a large cubical
Q19: An air-filled parallel-plate capacitor is connected to
Q19: If we use 67 W of power
Q23: If the electric flux through a closed
Q27: For the circuit shown in the figure,the
Q28: At a distance of 4.3 cm from
Q35: Consider a solenoid of length L,N windings,and
Q45: How much work is done by 3.00
Q52: A 905-g meteor impacts the earth at