In an electroplating process,copper (ionic charge +2e,atomic weight 63.6 g/mol) is deposited using a current of 10.0 A.What mass of copper is deposited in 10.0 minutes? Avogadro's number is 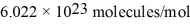 and e = 1.60 × 10-19 C.
and e = 1.60 × 10-19 C.
Definitions:
Issued Share Capital
Refers to the total value of a company's shares that have been sold to investors and are currently outstanding.
Business Combination Valuation Reserve
A reserve created during the accounting for a business combination to adjust the values of the combined entity's assets and liabilities to their fair values.
Acquisition Analysis
The process of evaluating the attractiveness and financial implications of a potential acquisition to determine its feasibility and benefits.
Deferred Tax Liability
A tax obligation that arises from temporary differences between the accounting and tax treatment of transactions, which will be paid in the future.
Q14: A charge Q is uniformly spread over
Q27: Light is incident normally from air onto
Q28: A hollow conducting spherical shell has radii
Q35: Which statements are true for an electron
Q43: The capacitive network shown in the figure
Q45: Two very long parallel wires in the
Q48: An ac circuit is shown in the
Q48: A multiloop circuit is shown in the
Q61: A certain source of sound waves radiates
Q77: What is the focal length of the