Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.63 nm.What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s, 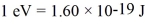 ,the Rydberg constant is R = 1.097 × 107 m-1)
,the Rydberg constant is R = 1.097 × 107 m-1)
Definitions:
Fear Of Failure
The fear that occurs when people are afraid of looking bad in front of others.
Success Feeling
The sense of achievement or fulfillment that comes from accomplishing goals or overcoming challenges.
Criticism Fear
The anxiety or apprehension of being judged, evaluated negatively, or receiving negative feedback.
Workplace Substance Policies
Regulations set by a company to manage or prohibit the use and abuse of drugs and alcohol within the workplace environment.
Q5: Light consisting of a mixture of red
Q10: A coating is being applied to reduce
Q19: The first step in the decision making
Q22: A hydrogen atom initially in the n
Q22: In the figure,a C-shaped conductor is in
Q27: A metallic sheet has a large number
Q28: Ionic bonding is due to<br>A) the sharing
Q34: To find the choice that provides the
Q56: Suppose you place your face in front
Q63: In the nuclear reaction<br>n + <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg"