A hydrogen atom makes a downward transition from the 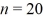 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
Definitions:
Personality
The combination of characteristics or qualities that form an individual's distinctive character.
Cognitive Psychologists
Scientists who study mental processes such as perception, memory, thought, and problem-solving.
Hopeless
A state of despair in which an individual perceives little or no possibility of overcoming a situation, often associated with depression.
Rumination
The compulsive focused attention on the symptoms of one's distress, and on its possible causes and consequences, as opposed to its solutions.
Q2: A nonrelativistic electron is accelerated from rest
Q3: In a multicriteria decision problem<br>A)it is impossible
Q3: When monochromatic light illuminates a grating with
Q7: For a maximization problem,the conservative approach is
Q9: What type of particle accelerator has two
Q10: What is the energy density in the
Q16: The excited state of a certain atom
Q40: Maximizing the expected payoff and minimizing the
Q41: A laser beam passes through a thin
Q74: Scandium, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6394/.jpg" alt="Scandium, Sc,decays