A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state.What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 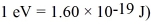
Definitions:
Pints
A unit of volume or capacity typically used for measuring liquids, equivalent to 1/8 of a gallon or approximately 473 milliliters in the United States.
Milliliters
A unit of volume in the metric system, equal to one thousandth of a liter.
Fluid
Fluid is a substance that can flow and take the shape of its container, encompassing liquids, gases, and plasmas.
Metric Tons
A unit of mass equal to 1,000 kilograms, or approximately 2,204.6 pounds, used worldwide in industrial and commercial applications.
Q1: A company seeks to maximize profit subject
Q2: The relationship d = 5000 - 25p
Q10: An electron is bound in an infinite
Q17: A photon of wavelength 29 pm is
Q19: An electric current through a tungsten filament
Q19: The eyepiece of a compound microscope has
Q26: Fold back the decision tree and state
Q32: Which of the following particles are made
Q33: Light of wavelength 500 nm illuminates a
Q34: If a beam of electromagnetic radiation has