A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state.What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J • s,h = 1.055 × 10-34 J • s, 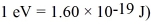
Definitions:
Major Heart Surgery
A significant surgical intervention on the heart or its major blood vessels to correct congenital defects, or to treat heart disease or damage.
Survival Surgery
A surgical procedure performed on animals for experimental purposes where the animal is expected to recover.
Presented Information
Information that is provided or made available to an individual or group for consideration or analysis.
Avoid Losses
A principle in behavioral finance and economics indicating people's tendency to prefer avoiding losses to acquiring equivalent gains, known as loss aversion.
Q13: The following are positioned in sequence: A
Q19: Light of wavelength 633 nm from a
Q22: If the density of the universe is
Q25: The decision making process includes implementation and
Q26: Fold back the decision tree and state
Q30: Calculate the ground state energy of a
Q33: A muonic hydrogen atom is a proton
Q38: Find the wavelength of the photon emitted
Q38: States of nature<br>A)can describe uncontrollable natural events
Q51: In a linear programming problem,the objective function