Consider the fusion reaction 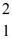 H +
H + 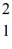 H +
H + 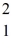 H →
H → 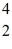 He +
He + 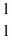 H +
H + 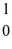 n
n
The atomic masses are: 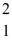 H: 2.01410 u
H: 2.01410 u 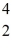 He: 4.00260 u
He: 4.00260 u 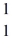 H,1.00783 u
H,1.00783 u 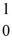 n: 1.008665 u
n: 1.008665 u
What mass of deuterium fuel, 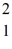 H,is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
H,is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Definitions:
Developing Countries
Nations with a lower level of industrialization, lower standard of living, and generally lower Human Development Index than developed countries.
Protective Equipment
Gear designed to safeguard individuals from physical, chemical, and biological hazards.
Chlorinated Hydrocarbons
A group of chemical compounds that contain carbon, hydrogen, and chlorine, often used in industrial applications.
Biologically Magnified
The process by which the concentration of a toxin increases as it moves through successive levels of a food chain.
Q4: Which one of the following decays CAN
Q6: Consider two different isotopes of the same
Q14: The first step in problem solving is<br>A)determination
Q27: An author has received an advance against
Q27: A video rental store has two video
Q31: Utility reflects the decision maker's attitude toward<br>A)probability
Q31: For a solid in which the occupation
Q34: Which of the following is a valid
Q35: What is the minimum speed needed by
Q41: Which of the following statements is NOT