The graph in the figure shows the electric field strength (not the field lines) as a function of distance from the center for a pair of concentric uniformly charged spheres.Which of the following situations could the graph plausibly represent? (There may be more than one correct choice.) 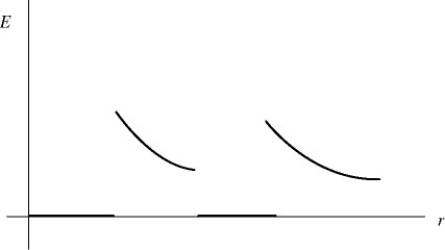
Definitions:
Allene
Organic compounds characterized by a cumulated diene system with two carbon-carbon double bonds.
Correct Hybridization
The mixing of atomic orbitals to form new hybrid orbitals, explaining the geometry of molecular bonding.
Atom
An atom is the smallest unit of matter that retains the identity of a chemical element, consisting of a nucleus surrounded by electrons.
Molecule
The smallest unit of a chemical compound that can exist; it consists of a group of atoms bonded together, representing the compound's chemical properties.
Q4: The phase angle of an LRC series
Q18: When two point charges are 2.0 cm
Q21: A metallic sphere of radius 5 cm
Q34: A uniform sign is supported against a
Q36: If the electric field is zero everywhere
Q37: An ac circuit is shown in the
Q38: A Carnot air conditioner operates between an
Q46: Two optically flat glass plates,16.0 cm long,are
Q49: A uniform solid disk of radius 1.60
Q56: In a double-slit experiment,if the slit separation