An ideal gas undergoes the process a→b→c→a shown in the pV diagram.The thermal energy transferred to the gas through heating gas in process a→b is 546 J,while in process the gas loses 62.0 J of thermal energy through heating.In process a→b there is - of work performed on the gas,while in process c→a 223 J of work is done on the gas.How much heat is gained by the gas in process c→a? 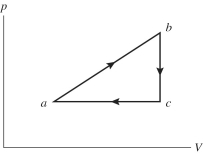
Definitions:
Long-term Memory
A type of memory responsible for the storage of information over an extended period, possibly a lifetime.
Rehearsal
The practice or process of repeating information, such as a performance or activity, to improve skill and memory.
Elongation
The process or state of extending or being elongated in physical length or duration.
Elaboration
A detailed explanation or development of an idea, concept, or theory.
Q2: Suppose that the Department of Energy develops
Q26: A small ball is tied to one
Q31: Three equal charges +Q are at three
Q35: Three point charges are located on the
Q46: What is absolute zero on the (a)Celsius
Q61: A 4.0-Ω resistor is connected with a
Q69: A 100-kg person sits on a 5-kg
Q93: The figure shows a pV diagram for
Q104: What is the <span class="ql-formula"
Q221: Two 4.0-Ω resistors are connected in parallel,and