An ideal gas undergoes the process a→b→c→a shown in the pV diagram.The thermal energy transferred to the gas through heating gas in process a→b is 546 J,while in process the gas loses 62.0 J of thermal energy through heating.In process a→b there is - of work performed on the gas,while in process c→a 223 J of work is done on the gas.How much heat is gained by the gas in process c→a? 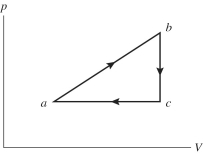
Definitions:
Labor-Force Participation Rate
The labor-force participation rate is the percentage of the working-age population that is either employed or actively seeking employment.
Bureau of Labor Statistics
A U.S. government agency responsible for collecting and analyzing economic data related to labor market activity, working conditions, and price changes.
True Unemployment Rate
A measure of the actual number of unemployed individuals, considering those who have stopped looking for work or are underemployed.
Q4: A croquet mallet balances when suspended from
Q15: Initially,a small 2.0-kg rock is whirling at
Q16: Three objects of masses 1.0 kg,2.0 kg,and
Q50: An ideal parallel-plate capacitor consists of two
Q53: As shown in the figure,a given
Q72: A 50.0-N brick has the following measures:
Q100: A piece of iron of mass 0.12
Q108: Suppose a uniform solid sphere of mass
Q111: When a potential difference is applied
Q190: The following three appliances are connected in