Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?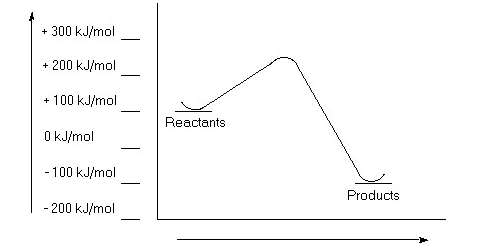
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
Definitions:
Positive Culture
A workplace environment that promotes values such as respect, teamwork, and open communication, leading to higher employee morale and productivity.
Behavioral-Based
Pertains to assessment or interventions focusing on specific behaviors rather than traits or capabilities, often used in the context of performance management and hiring.
Job Satisfaction
The level of fulfillment, happiness, and contentment an employee feels towards their job.
Organizational Efficiency
The ability of an organization to implement its strategies and achieve its goals with minimum resources and waste.
Q3: When calculating the NPV, what discount rate
Q9: Properly connect three monomeric acrylonitrile units (the
Q11: Which of the following is able to
Q23: Which of the following is the holding
Q27: Which of the following is true when
Q31: In a chemical bond, which atom gets
Q52: In the equation N<sub>2</sub> + 2 O<sub>2</sub>
Q59: The formula C<sub>2</sub>H<sub>6</sub>O may be attributed to
Q74: An aqueous solution of HCl is called
Q94: At equilibrium, the "amount of product" present