Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?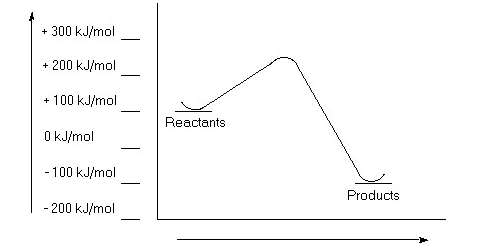
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
Definitions:
Organizational Citizenship Behaviour
Refers to voluntary actions employees take to help others and benefit the organization, beyond their job requirements.
Low Risk
Describes situations, investments, or actions that have a minimal chance of negative outcome or loss.
Retroactive Interference
The disruptive effect of new learning on the recall of old information.
Memory Retrieval
The process of recalling information stored in the brain.
Q1: Which of the following is not a
Q12: What is the molarity of 3.68 mL
Q15: Assuming that the pressure and quantity of
Q23: Identify the reducing agent in the following
Q29: Which of the following is not a
Q44: The pressure of 0.500 atm is equal
Q66: 5.82 g isopropyl alcohol yield 7.001 g
Q81: Which of the following is water soluble?<br>A)CCl<sub>4</sub><br>B)C<sub>2</sub>H<sub>5</sub>OH<br>C)C<sub>8</sub>H<sub>18</sub><br>D)CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub>
Q92: The molarity of a solution that contains
Q145: One mole of carbon monoxide has the