Calculate the standard free energy of formation of mercury(II) oxide at 298 K,given 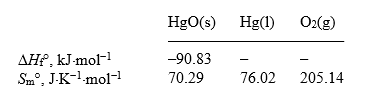
Definitions:
Production Process
The method or series of actions taken to manufacture a product, from the acquisition of raw materials to the final product.
Present Value
Today's value of a future money amount or sequence of cash flows, determined by a certain rate of return.
Initial Investment
The initial amount of money invested in a project or venture.
Financial Break-Even
The point at which total revenues equal total costs and expenses, leaving no net gain or loss.
Q20: Which of the following has the smallest
Q30: Superconductivity is the loss of all electrical
Q33: A CD player and its battery together
Q37: Which of the following reactions has
Q54: Calculate the standard entropy of vaporization of
Q59: All of the following are strong bases
Q64: Calcium cyanamide reacts with H<sub>2</sub>O(g)at 150<sup>
Q67: Tetrabromomethane has a higher boiling point than
Q68: Which of the following statements is true?<br>A)A
Q84: The boiling points of the Group 14