Multiple Choice
The phase diagram for a pure substance is given below.The solid sublimes 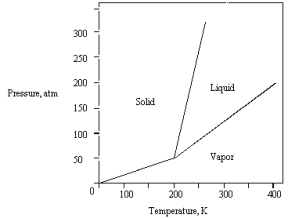
Definitions:
Related Questions
Q3: Which of the following has the highest
Q6: Consider the following cell: Zn(s)|Zn<sup>2+</sup>(aq,0.100 M)m
Q6: The equilibrium constant K for the
Q12: The pH of 0.30 M CH<sub>3</sub>NH<sub>2</sub>(aq)is 12.0.Therefore,the
Q16: Resistance in a material is defined
Q27: At equilibrium,Q = K and
Q31: A plot of ln(vapor pressure)versus 1/T for
Q34: A 0.479-g sample of nitrogen,oxygen or
Q58: In a closed vessel containing water the
Q88: Give <span class="ql-formula" data-value="\Delta"><span class="katex"><span