The Phase Diagram for Carbon Dioxide Is Given Below C,at 1 Atm and Room Temperature
A)solid Carbon Dioxide Is
The phase diagram for carbon dioxide is given below. 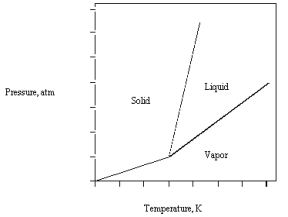
If the triple point is at 5.1 atm and -56 C,at 1 atm and room temperature
Definitions:
Q10: The pH of 0.50 M HNO<sub>2</sub>(aq)is 1.8.Therefore,the
Q11: Which of the following pairs have a
Q13: Molecular solids are held together by weak
Q23: Consider the compounds <br>PCl<sub>5</sub>(g),HCN(g),CuO(s),NO(g),NH<sub>3</sub>(g),and SO<sub>2</sub>(g).<br>Which compound will
Q24: If the rate of reaction increases by
Q29: Sulfur dioxide reacts with oxygen gas
Q30: Consider the following reaction at a certain
Q50: Consider the reaction PCl<sub>5</sub>(g) →PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)<br>At a
Q66: Given: 2P(g)+ 3Cl<sub>2</sub>(g) →2PCl<sub>3</sub>(g)K = 15.0 PCl<sub>3</sub>(g)+
Q79: The galvanic cell shown above uses the