Consider the reaction NO(g) + 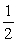 O2(g) NO2(g)
O2(g) NO2(g)
If H = -56.52 kJ and S = -72.60 J.K-1 at 298 K,what is the equilibrium constant for the reaction at 298 K?
Definitions:
Warm Current
A current of warmer ocean water that moves away from the equator towards the poles, influencing the climate of the regions it flows through.
Ocean Currents
Streams of seawater that move through the earth's oceans in a regular pattern, influenced by wind, salinity, and temperature differences.
Cold Current
A mass of seawater that moves from higher to lower latitudes, typically colder than the surrounding waters and influencing local climates.
Arid Lands
Regions characterized by a severe lack of available water, to the extent of hindering or preventing the growth and development of plant and animal life.
Q15: What is the conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>-</sup>
Q17: Calculate the average H-S bond enthalpy in
Q18: The standard free energy of formation of
Q29: What is the major component of pewter?<br>A)Antimony<br>B)Zinc<br>C)Tin<br>D)Copper<br>E)Iron
Q29: The reaction 2C(s)+ 2H<sub>2</sub>(g) <span class="ql-formula"
Q35: The critical temperature of N<sub>2</sub> is -147<sup>o</sup>C.Nitrogen
Q56: A buffer solution contains 0.0200 M acetic
Q65: All of the following are Lewis bases
Q68: The energy levels of two particle in
Q143: The equation for the hydrometallurgical extraction