Given: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
K
Calculate the equilibrium constant for the following reaction.
2NH3(g) + 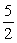 O2(g) →2NO(g) + 3H2O(g)
O2(g) →2NO(g) + 3H2O(g)
Definitions:
Specialized Professionals
Individuals with expertise in a particular area or field, often requiring specific education or training.
Service Quality
A measure of how well a delivered service matches customer expectations, often evaluated through dimensions like reliability, responsiveness, assurance, empathy, and tangibles.
Gap Analysis
A method of assessing the differences between actual performance and potential or desired performance in an organization, often used to identify areas for improvement.
Organizational Delivery
The method or process through which a company provides its products or services to its customers.
Q1: Galvanized iron is protected from corrosion because
Q13: In the Daniell cell,the anode is zinc
Q15: Consider the compounds <br>PCl<sub>5</sub>(g),HCN(g),CuO(s),NO(g),NH<sub>3</sub>(g),and SO<sub>2</sub>(g).<br>Which compound will
Q20: At what general temperature are interstitial carbides
Q48: If the standard reaction enthalpy for
Q54: Which of the following 0.10 M aqueous
Q62: A cubic close-packed structure has<br>A)A coordination number
Q83: How is boron nitride related to graphite?<br>A)It
Q88: Calculate the equilibrium constant for the reaction
Q93: How do alloys solidify and melt?<br>A)At precise