Consider the reaction NO(g) + 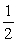 O2(g) NO2(g)
O2(g) NO2(g)
If H = -56.52 kJ and S = -72.60 J.K-1 at 298 K,what is the equilibrium constant for the reaction at 298 K?
Definitions:
Regulate
Means to control or maintain the rate or speed of a process or system by making adjustments or imposing controls.
Reflective Listening
A communication technique involving understanding a speaker's message and then offering it back to the speaker to confirm the message has been understood correctly.
Active Listening
The process of fully concentrating, understanding, and responding during the course of a communication.
Developmental Feedback
Constructive comments or assessments aimed at fostering growth, learning, and improvement.
Q2: Consider the reaction <br>CuSO<sub>4</sub>(s) <span class="ql-formula"
Q15: What is the conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>-</sup>
Q28: Which pair of metals will dissolve in
Q42: Mercury,one of the first materials in which
Q45: A CD player and its battery together
Q49: For CaCl<sub>2</sub>,the enthalpies of hydration and
Q60: Which has the higher boiling point,HCl or
Q73: If the pK<sub>a</sub> of acetic acid is
Q91: What is the change in entropy when
Q93: The pH of 0.10 M pyridine(aq)is 9.13.What