Multiple Choice
Given: SO2(g) →O2(g) + S(s)
Kc = 2.5 * 10-53
SO3(g) →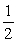 O2(g) + SO2(g)
O2(g) + SO2(g)
Kc = 4.0 * 10-13
Calculate Kc for the reaction
2S(s) + 3O2(g) →2SO3(g)
Definitions:
Related Questions
Q4: Consider the reaction <br>NOBr(g) <span class="ql-formula"
Q16: A certain liquid has a meniscus that
Q18: Strong acids are leveled in water to
Q45: A CD player and its battery together
Q47: Using the cell shown above,A is
Q51: How do the saline hydrides react in
Q59: All of the following are strong bases
Q64: The molar heat capacity of a monatomic
Q79: Given: <br>2A(g)+ B(g)+ C(g) <span class="ql-formula"
Q89: The phase diagram for CO<sub>2</sub> is