At 600 C,the equilibrium constant for the reaction 2HgO(s) → 2Hg(l) + O2(g)
Is 2.8.Calculate the equilibrium constant for the reaction 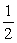 1/2O2(g) + Hg(l) → HgO(s)
1/2O2(g) + Hg(l) → HgO(s)
Definitions:
Divergent Thinking
An approach to problem-solving that involves generating multiple solutions or ideas.
Verbal Abilities
The capacity to use language effectively and understand its nuances, including vocabulary, grammar, and expression.
Gardner's Multiple Intelligences
A theory proposed by Howard Gardner, suggesting that individuals possess differing kinds of intelligences, including verbal-linguistic, logical-mathematical, spatial, musical, bodily-kinesthetic, interpersonal, intrapersonal, and naturalistic.
Bodily-Kinesthetic
A type of intelligence that involves the capacity to manipulate objects and use physical skills effectively, as conceptualized by Howard Gardner's theory of multiple intelligences.
Q1: How does an electron well form in
Q8: Of the following,which is the strongest acid?<br>A)CH<sub>3</sub>COOH
Q14: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,has K<sub>a1</sub> =
Q20: The phase diagram for a pure substance
Q22: The HBr synthesis is thought to involve
Q31: Which one of the following statements
Q33: The equation that represents K<sub>a2</sub> for sulfurous
Q35: Which of the following aqueous solutions gives
Q39: What is the pH at the stoichiometric
Q94: Where is the tensile strength of carbon