Given: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
K
Calculate the equilibrium constant for the following reaction.
2NO(g) + 3H2O(g) →2NH3(g) + 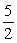 O2(g)
O2(g)
Definitions:
Law Firm
A business enterprise formed by one or more lawyers to engage in the practice of law, providing legal services to clients.
Corporation
A legal entity that is separate and distinct from its owners, which can own property, enter into contracts, sue and be sued.
Document-Creation Skills
The ability to create written documents effectively and efficiently, often involving the use of computer applications and understanding of formatting and content organization.
Legal Research
The process of identifying and retrieving information necessary to support legal decision-making, including statutes, case law, and legal precedents.
Q12: What are the coordination numbers of calcium
Q27: What is the pH at the half-stoichiometric
Q48: Which of the following metals would be
Q54: Brass is an example of what kind
Q61: The following compounds are available as 0.10
Q86: The solubility of all except which the
Q90: The reaction CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q93: How do alloys solidify and melt?<br>A)At precise
Q93: A cell that uses bromine to oxidize
Q120: When metallic hydrides are heated,they<br>A)decompose explosively to