Given: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
K
Calculate the equilibrium constant for the following reaction.
2NH3(g) + 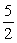 O2(g) →2NO(g) + 3H2O(g)
O2(g) →2NO(g) + 3H2O(g)
Definitions:
Perceived Partner
An individual's subjective understanding or assessment of their romantic or significant other.
Uniqueness
The quality of being one of a kind or unlike anything else, often valued for standing out from the norm.
Transgressions
Acts that go against a law, rule, or code of conduct; violations.
Self-Image
An individual's conception of themselves or their identity.
Q8: Consider the following cell:<br>Zn(s)|Zn<sup>2+</sup>(aq,0.200 M)m H<sup>+</sup>(aq,?)|H<sub>2</sub>(g,1.00
Q17: What is a eutectic point?<br>A)0an alloy's point
Q22: If the ratio of the radius of
Q27: When three phases are in mutual equilibrium,such
Q41: the triple point for water,4.6 Torr
Q63: At 600<sup> <span class="ql-formula" data-value="\circ"><span class="katex"><span
Q64: The solubility of silver bromide is greater
Q71: What is the pH of an aqueous
Q76: Consider the phase diagrams for water and
Q92: The concentration-time dependence for a first-order reaction