The following compounds are available as 0.10 M aqueous solutions. 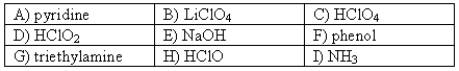 Which two solutions could be used to prepare a buffer with a pH of ~ 7?
Which two solutions could be used to prepare a buffer with a pH of ~ 7?
Definitions:
Depreciation
The systematic allocation of the cost of a tangible asset over its useful life, reflecting the decrease in value due to wear and tear, aging, or obsolescence.
EAC Method
Equivalent Annual Cost Method, a decision-making tool used for evaluating the cost-effectiveness of projects with differing lifespans.
Mutually Exclusive
Situations or decisions that cannot occur simultaneously; choosing one option means the others cannot be chosen.
Projects
Specific tasks or programs undertaken to achieve goals within a timeframe, often involving research or design efforts.
Q2: Calculate the number of moles of
Q5: The nitrate ion can act as a
Q14: The amino acid alanine,HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,has K<sub>a1</sub> =
Q24: The formation of solid calcium oxide from
Q28: Calculate the change in entropy of
Q44: For the reaction <br>2SO<sub>3</sub>(g) <span class="ql-formula"
Q54: Calculate the standard entropy of vaporization of
Q72: All of the following 0.1 M aqueous
Q87: Given: A <span class="ql-formula" data-value="\rightarrow"><span
Q92: For NH<sub>3</sub>,pK<sub>b</sub> = 4.74.What is the pH