The curve for the titration of 50.0 mL of 0.0200 M C6H5COOH(aq)with 0.100 M NaOH(aq)is given below.What are the main species in the solution after 7.5 mL of base have been added? 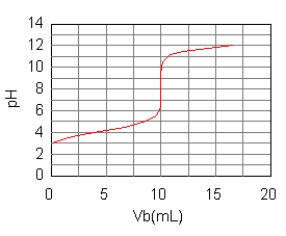
Definitions:
Suctioning
The process of removing mucus and other fluids from a patient's airway using a suction device to maintain airway patency.
Ear Irrigation
A medical procedure used to remove earwax buildup or foreign objects from the ear canal with a stream of fluid.
Vital Signs
are the measurements of the body’s most basic functions, including temperature, blood pressure, pulse, and respiratory rate.
Seizure Precautions
Measures taken to protect someone from injury during a seizure, such as removing nearby hazards and providing a safe place to lie down.
Q5: For the reaction N<sub>2</sub>O<sub>4</sub>(g) →2NO<sub>2</sub>(g)<br>Which of
Q19: Consider the following cell:<br>Zn(s)|Zn<sup>2+</sup>(aq,0.10 M)m Cu<sup>2+</sup>(aq,0.10 M)|Cu(s)<br>At
Q24: The vapor pressure of water above
Q25: At the stoichiometric point in the titration
Q37: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q45: Phosphorus(V)oxide reacts with water to produce<br>A)H<sub>3</sub>PO<sub>2</sub>.<br>B)H<sub>3</sub>PO<sub>4</sub>.<br>C)HPO<sub>3</sub>.<br>D)H<sub>3</sub>PO<sub>3</sub>.<br>E)H<sub>3</sub>PO<sub>2</sub><sup>-</sup>.
Q57: Consider the phase diagrams for water and
Q59: All of the following are strong bases
Q83: Calculate the [OH<sup>-</sup>] in an aqueous solution
Q106: Which of the following is an interstitial