Use the following diagram of a cell to answer questions 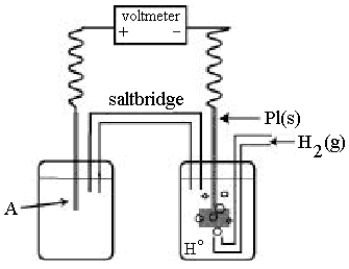
-Using the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,which half-reaction occurs in the left-hand cell compartment?
Definitions:
Average Total Cost
The per unit cost of production, calculated by dividing the total costs (fixed and variable) by the total quantity of output produced.
Economic Profits
The excess of a firm's total revenues over its opportunity costs, reflecting profitability beyond the normal return on investment.
Competitive Price-searcher Market
A market in which firms have some control over prices because their products are differentiated, but they still face competition and must search for competitive pricing strategies.
Zero Economic Profit
A situation where a firm's total revenue equals its total costs, including both explicit and implicit costs, indicating no supernormal profit.
Q31: The mineral spondumene contains long,straight chain silicates
Q34: Choose the effective pH range of an
Q39: Calculate the equilibrium constant for the reaction
Q47: What is the equilibrium constant for the
Q48: Which of the following is the weakest
Q51: How do the saline hydrides react in
Q53: Consider the phase diagram for sulfur in
Q65: At 25<sup> <span class="ql-formula" data-value="\circ"><span class="katex"><span
Q83: For the complex ion [Co(en)(NH<sub>3</sub>)<sub>2</sub>(OH<sub>2</sub>)Cl]<sup>2+</sup>,there are four
Q84: The standard voltage of the cell