Use the following to answer questions 55-58: 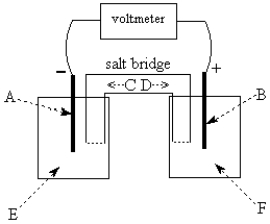
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Petty Cash Fund
A small amount of cash kept on hand for minor, immediate expenses.
Cash Short
An accounting term referring to a situation where the actual physical cash differs from the expected amount, typically indicating a shortfall.
Missing Funds
A financial discrepancy where funds are unaccounted for due to errors, theft, or fraud.
Petty Cash Fund
A petty cash fund is a small amount of cash kept on hand for making immediate, low-cost expenditures, reducing the need for writing checks or using credit transactions.
Q22: The HBr synthesis is thought to involve
Q32: The equilibrium constant,K,for the reaction 2HgO(s) →
Q40: For the equilibrium N<sub>2</sub>O<sub>4</sub>(g) →2NO<sub>2</sub>(g),plot,on the same
Q43: Which of the following indicators would be
Q47: A sample of carbon from the Lascaux
Q62: A complex that is the subject of
Q71: the van't Hoff i of HBr,HCl,and HF
Q77: For the reaction <br>HO(g)+ H<sub>2</sub>(g) <span
Q89: All the halogens exist as diatomic molecules
Q107: Consider the following possible reactions:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1441/.jpg" alt="Consider