Lithium and nitrogen react to produce lithium nitride: 6 Li(s) + 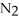 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of 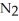 are needed to react with 0.550 mol of lithium?
are needed to react with 0.550 mol of lithium?
Definitions:
Physically Weak
Definition: Having low physical strength or vitality, often resulting in difficulty performing tasks requiring physical effort.
Recover From Illnesses
The process of regaining health and wellness after suffering from a disease or medical condition.
Quality Of Relationships
The perceived value and depth of interpersonal connections, often influencing personal satisfaction and emotional well-being.
Staving Off Loneliness
Methods or strategies used to cope with or reduce feelings of loneliness.
Q15: Calculate the root mean square velocity of
Q15: How much calcium is contained in 35.0
Q27: A 21.8 g sample of ethanol (C<sub>2</sub>H<sub>5</sub>OH)is
Q78: Describe the difference between an atomic element
Q87: The density of nitric oxide (NO)gas at
Q96: Give the name for KHSO<sub>3</sub>.<br>A) monopotassium bisulfide<br>B)
Q117: How many moles of Kr are contained
Q161: Which of the following is a precipitation
Q168: Define an atom.
Q211: What volume of a 0.540 M NaOH