Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6 Li(s) + 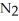 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of lithium are needed to produce 0.31 mol of 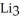 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
Understand the concept of leadership in organizational efficiency and its effects.
Recognize the influence of situational factors on employee motivation and performance.
Understand the concepts and types of sexual scripts and their societal, cultural, and individual influences.
Recognize the influence of peers, media, and cultural norms on sexual attitudes and behaviors.
Definitions:
Related Questions
Q1: Which of the following is considered a
Q8: Which of the following elements is a
Q23: How many moles are there in 1.50
Q56: An endothermic reaction has<br>A) a negative ΔH,
Q68: Which of the following is the correct
Q75: Sodium metal and water react to form
Q81: An atom of 131<sub>I contains _ neutrons.</sub><br>A)
Q129: Energy that is associated with the position
Q151: In which set do all elements tend
Q229: Which of the following compounds is soluble