Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 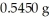 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Publicity Tools
Various methods and mediums used to generate public attention or news coverage for a product, event, or organization, often without direct payment.
Nonpersonal Presentation
A marketing strategy that involves the promotion of products or services without direct human interaction, typically through media and digital channels.
Direct Cost
Expenses that can be directly attributed to the production of specific goods or services, such as raw materials and labor costs.
Implementation Process
The series of actions taken to put a plan or strategy into action.
Q2: Use the standard reaction enthalpies given below
Q10: Identify barium hydroxide.<br>A) strong electrolyte, strong base<br>B)
Q23: Which one of the following compounds behaves
Q47: Which one of the following compounds is
Q50: The volume of a gas is proportional
Q72: Give the correct formula for aluminum sulfate.<br>A)
Q111: Give the name for KHSO<sub>3</sub>.<br>A) monopotassium sulfite<br>B)
Q138: The law of _ states that energy
Q168: Write the name for Mg<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>.<br>A) magnesium(III) phosphite<br>B)
Q199: How many milliliters of 0.200 M FeCl<sub>3</sub>