Lithium and nitrogen react to produce lithium nitride: 6 Li(s) + 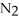 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of 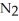 are needed to react with 0.550 mol of lithium?
are needed to react with 0.550 mol of lithium?
Definitions:
Demand
An economic principle referring to the consumer's desire and willingness to pay for a specific quantity of a good or service at a given time.
Book
A published material that features pages bound together on one side and encased within protective covers.
Marginal Revenue
The additional income received from selling one more unit of a product or service.
Demand Function
A mathematical representation showing the relationship between the quantity of a good that consumers are willing to buy and its price, along with other factors like income and prices of related goods.
Q11: Define molar heat capacity.<br>A) the quantity of
Q20: Give an example of an halogen.
Q26: Determine the name for Br<sub>2</sub>O.<br>A) bromine oxide<br>B)
Q34: How many atoms are in 2.50 moles
Q35: Choose the statement below that is TRUE.<br>A)
Q45: Calculate the change in internal energy (ΔE)for
Q53: Convert 3.3 μm to meters.<br>A) 3.3 ×
Q81: A sample of copper absorbs 43.6 kJ
Q104: If a sample of 0.29 moles of
Q229: Which of the following compounds is soluble