Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6 Li(s) + 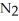 (g) → 2 Li3N(s)
(g) → 2 Li3N(s)
How many moles of lithium are needed to produce 0.31 mol of 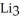 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
Definitions:
Equity-Financed
This refers to the method of funding a business or purchasing assets using primarily equity rather than debt.
Merger Premium
The additional amount that a company pays over the current market value of a target company during an acquisition, reflecting the anticipated synergy value.
Incremental Value
The additional value created by a new investment or project, compared to the scenario without it.
Equity-Financed
A method of raising capital whereby a company issues new shares of stock and receives fresh capital in return but does not incur debt.
Q14: Consider the following balanced reaction.How many grams
Q21: What precipitate is most likely formed from
Q36: Which of the following solutions will have
Q39: Write the name for SnS.<br>A) stannous sulfate<br>B)
Q62: Give the net ionic equation for the
Q75: The average atomic mass for silver is
Q92: Which of the following compounds is vinegar?<br>A)
Q143: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q231: According to the following reaction,what volume of
Q238: Identify the oxidation state of H in