Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 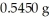 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
Definitions:
Problems
Issues or obstacles that need to be solved or overcome.
Scientific Charity
An approach to philanthropy that emphasizes the use of research, evidence, and data to inform charitable activities and maximize impact.
Change Agent
An individual or group that actively facilitates, implements, and supports change within an organization or community.
Social Innovation
The development and implementation of new, effective solutions to social problems or needs that create social value and involve the collaboration of multiple stakeholders.
Q14: How many grams of NO<sub> </sub>gas are
Q18: Why do the isotopes of the same
Q22: Hydroelectric power plants produce nearly _ percent
Q45: What is the total volume of the
Q55: How many liters of oxygen gas can
Q92: Which statement is FALSE?<br>A) An exothermic reaction
Q101: Which of the following is NOT a
Q127: What is the concentration of FeCl<sub>3</sub> in
Q129: How many moles of NF<sub>3</sub> contain 2.55
Q165: What is the molarity of a potassium