The combustion of titanium with oxygen produces titanium dioxide: Ti(s) + 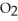 (g) →
(g) → 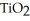 (s)
(s)
When 1.720 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00°C to 80.10°C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJ/K.The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ/mol.
Definitions:
Journal
A record where all financial transactions are initially entered, which may later be posted to a ledger.
Company's Records
Company's records comprise all documents, books, papers, and financial statements that depict the business transactions and financial status of a company.
Credit Memo
A document issued by a seller to a buyer, reducing the amount that the buyer owes to the seller under the terms of an existing invoice.
Bank Service Charges
Fees charged by banks for account services, transactions, and maintenance.
Q10: Identify the color that has a wavelength
Q38: Use Lewis theory to determine the chemical
Q52: Give the number of valence electrons for
Q98: What is the molar concentration of lithium
Q108: A 50.0-g sample of liquid water at
Q110: A 4.50-g sample of copper metal at
Q121: A syringe contains 589 mL of CO
Q137: Give the number of core electrons for
Q154: A gas mixture contains Rn,He and N<sub>2</sub>.What
Q237: A solution is prepared by adding 1.70